Outputs
D6.1 - Website Twitter and LinkedIn accounts live
17/10/2025Public deliverable.
The document describes the motivation behind the concept of the website and the objectives as a key tool for dissemination and communication actions. The document provides a description of the public site, defining also the social media tools used, such us Twitter and LinkedIn.
Read the deliverable here.
D4.2 - Network-wide training events report - R1
17/10/2025Public deliverable
The document describes the activities carried out during the first D-Carbonize Workshop & kick-off meeting co-organized by ICIQ and RUG (24-25 January, Tarragona, Spain).
Read the deliverable here.
D4.3 - Network-wide training events reports - R2
17/10/2025Public deliverable.
The document describes the activities carried out during the second D-Carbonize Workshop organized by UISCR-CNRS (14-15 October, Rennes, France).
Read the deliverable here.
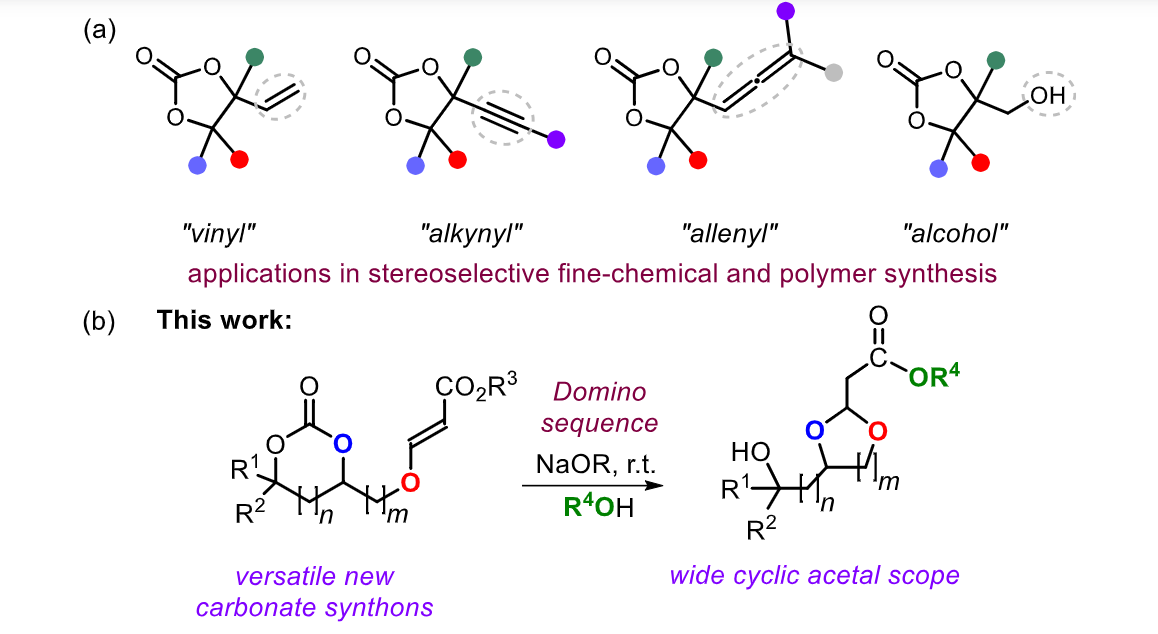
Domino Synthesis of Functionalized Cyclic Acetals From Organic Carbonates
04/12/2025Natalia Kulbacka - DC2
We report a method for the base-mediated transformation of ether-tethered acrylic ester-based cyclic carbonates into functionalized cyclic acetals. The protocol builds on the use of hydroxyalkyl-substituted cyclic carbonates that undergo an oxa-Michael addition reaction in the presence of alkyl propiolates thereby forging (E)-configured acrylic ether intermediates. The scope of the reaction involves the use of both five- and six-membered cyclic carbonates, and correspondingly, both five- andsix-membered cyclic acetals can be prepared. The amount of reagents, the purification method, and the type of ester substrate all contribute to the efficiency of the transformation. Mechanistic control reactions point at the intermediacy of an alkoxide that induces an intramolecular Michael addition onto the acrylic double bond following alkoxide-mediated formation of both an alcohol and ester in the final product. These functional groups, among others, further enable easy diversification of acetal-based synthons.
Click here to read the article.
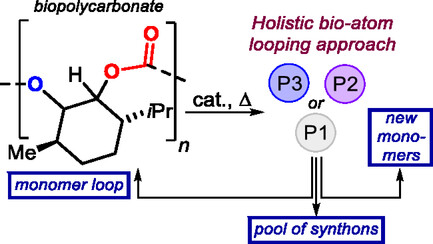
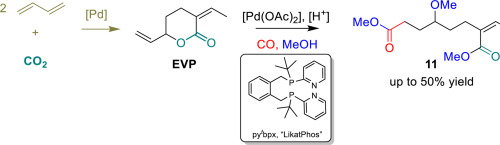
Enabling a Diversity-Oriented Catalytic Atom Looping of a Biobased Polycarbonate
06/10/2025Click here to read the article.
.png)
_1760689135.png)
_1760689697.png)